Introduction
Penetration testing performs an important function in FDA 510 k compliance by diligently detecting and assessing adverse cyber threats in healthcare products. This permits makers of the companies to show to the FDA that their products have strong cybersecurity features in place to safeguard the patient and integrations of the data, which is important for a successful 510(k) submission and industry clearance. Significantly, it ensures that a healthcare device and its products can resist simulated attacks and reduce the risks before they become available in the marketplace.
What is FDA 510(k) compliance in penetration testing?
A crucial security evaluation procedure for healthcare equipment for pursuing FDA authorization via the 510(k) channel is FDA 510(k) Compliance Penetration Testing. In order to find and assess the possible flaws in the device’s applications, this particular assessment replicates the actual cyberattack.
Latest Penetration Testing Report

Key points on the penetration testing in FDA 510(k) compliance
- Finding threats and vulnerabilities
FDA 510 k performance testing seeks flaws in the code, applications, network links, and interfaces of medical equipment to find potential routes of access for hostile actors.
- Risk evaluation
Through the simulation process of actual hacking attempts, penetration testing enables companies to assess the seriousness of risks found and rank remedial operations according to their possible influence on patient welfare.
- Approval of security controls
The Penetration testing confirms whether current safety policies, such as passwords, identification, and entry oversight, are successfully reducing cyber threats
- Applying rules and regulations
A comprehensive FDA 510k guidance procedure recorded in a detailed evaluation shows the Food and Drug Administration that an organization has taken the required actions to tackle safety concerns and adhere to its regulations.
- Need for premarket delivery
Nowadays, companies have to provide proof of thorough vulnerability testing, possibly particularly penetrating test results, within the context of a 510k compliance filing.
A few Beneficial concepts of penetration testing for FDA 510(k)
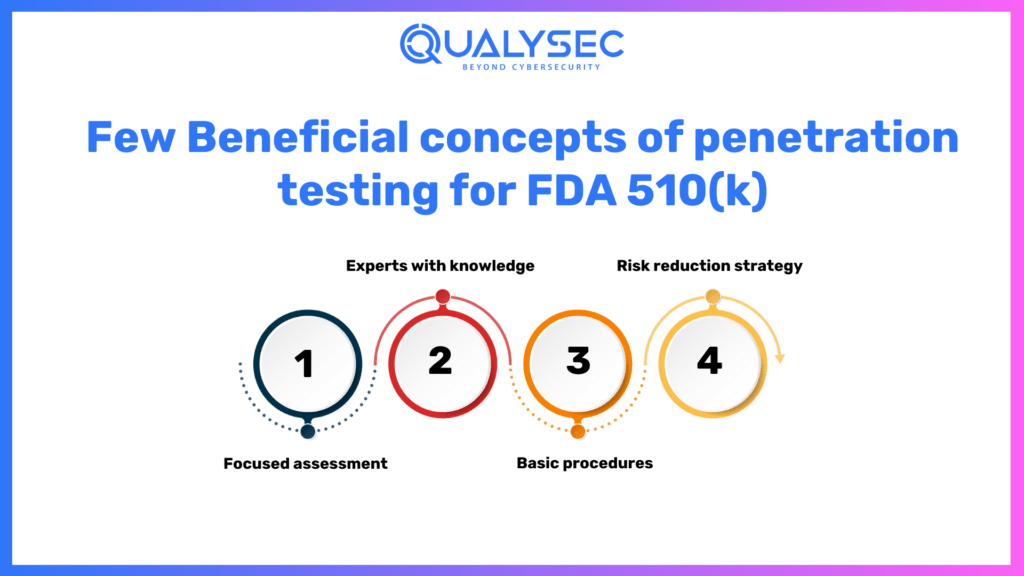
- Focused assessment:
Given the product’s planned usage, functioning setting, and probable attacks, vulnerability testing needs to be customized for each gadget.
- Experts with knowledge:
It is vital to work with an FDA-certified cybersecurity organization that specializes in healthcare device protection to guarantee a thorough and precise evaluation.
- Basic procedures:
Sticking to recognized guidelines such as AAMI TIR57 and NIST SP 800-115 allows for thoroughness and integrity in the testing approach.
- Risk reduction strategy:
The FDA 510(k) submission process must include a written strategy for addressing identified risks and implementing corrective actions.
The Need to Persuade Penetration Testing for FDA 510(k) Compliance
Handling challenging requirements by following the FDA 510 k premarket approval stage and Post-Market Guidance’s many cyberspace obligations, including risk assessment, safety evaluation, risk analysis, and reporting
Safeguarding confidential or proprietary information And protecting ideas, sensitive data, and medical information against accidental or illegal release.
Providing secure interfaces overseeing and protecting an intricate and varied network of linked healthcare systems and equipment.
The changing environment of cyber dangers, adjusting to and reducing the possibility associated with the constantly changing security threats environment that targets the medical field
How does penetration testing enable FDA 510(k) Compliance?
- Discovering risks that are undiscovered or concealed.
- Determining any vulnerabilities in healthcare applications or equipment and the framework that supports them that a criminal might take advantage of.
- Verifying to evaluate safety protocols.
- Evaluate how well the current cybersecurity protections against focused intrusion efforts or current risks are working.
- Integrating with the norms for cybersecurity and FDA guidelines.
- Making certain the most recent privacy regulations plus FDA advice are applied correctly.
- Setting priorities and recording all you did to reduce hazards.
- Learning the flaws is most important so that you can organize correction efforts and spend funds efficiently.
- Demonstrating the safety precautions and enhancements with ease.
Conclusion
The business can stay on pace for authorization by obtaining the guidance of a seasoned cybersecurity company for the application and healthcare device protection plan. With the 510(k) premarket and postmarket applications, the company will collaborate with professionals knowledgeable about the FDA’s regulations.
They will conduct the evaluation and pen testing in accordance with FDA compliance. This method is extremely effective and precise for determining risk factors and fixing problems so that you can satisfy FDA requirements.
It’s possible to confidently submit your 510(k) following a preliminary evaluation, penetration testing, and plan creation. The way you contributed to security, however, continues beyond here. Frequent risk evaluations and pen tests will help companies stay up to date with FDA regulations.
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
Ensure your healthcare solution is globally compliant.
Qualysec helps you meet HIPAA, FDA, ISO, and more. Contact us today!





















![Top 20 Network Security Companies in USA [2025 Updated List]](https://qualysec.com/wp-content/uploads/2025/05/Top-20-Network-Security-Companies-in-USA-2025-Updated-List-scaled.jpg)











































































































































































































































































































































































































0 Comments