The medical device sector is changing quickly as connectedness and innovation push the limits of what is achievable in healthcare. But as things advance, new regulations are required to guarantee the security and effectiveness of medical equipment. In this context, the Food and Drug Administration (FDA) of the United States plays a vital role. It sets criteria and FDA guidance for medical device security that are required to adhere to.
The FDA revised its cybersecurity recommendations for medical devices, by highlighting the significance of including strong security measures at an earlier stage of the product development lifecycle. We explore the main features of these new rules in this blog post, by giving medical device product teams the knowledge they require to handle Premarket Submissions under the updated FDA Cybersecurity Guidance.
Understanding FDA Guidance for Medical Device Security
Medical device security is concerned with securing devices like pacemakers, insulin pumps, and monitors against unauthorized access and tampering. Moreover, this helps to protect patient safety and data integrity so that private information is not compromised due to data breaches. Security measures include encryption, authentication, software updates, and penetration testing. Additionally, by keeping these devices safe, healthcare providers can establish trust with patients while upholding the credibility of medical data.
FDA Guidance Overview
The FDA Cybersecurity Guidance on Medical Device Security defines the key regulations for ensuring the security and integrity of medical devices in a more connected healthcare environment including FDA guidance for medical device security. Additionally, it focuses on risk assessment, design controls, vulnerability management, software and patch management, information sharing, collaboration, implementation, and compliance. These elements combined are a response to the dynamic problems of cyber security in medical technology. Hence, by implementing this guidance manufacturers can empower device resilience to potential risks, assure data protection, and maintain the loyalty and reliability of medical devices.
Key Components of FDA Guidance
 The components of FDA guidance for medical device security imply that the attention is to provide, guarantee, and sustain the safety, effectiveness, and reliability of medical devices or software in healthcare settings. Here’s a breakdown of each component:
The components of FDA guidance for medical device security imply that the attention is to provide, guarantee, and sustain the safety, effectiveness, and reliability of medical devices or software in healthcare settings. Here’s a breakdown of each component: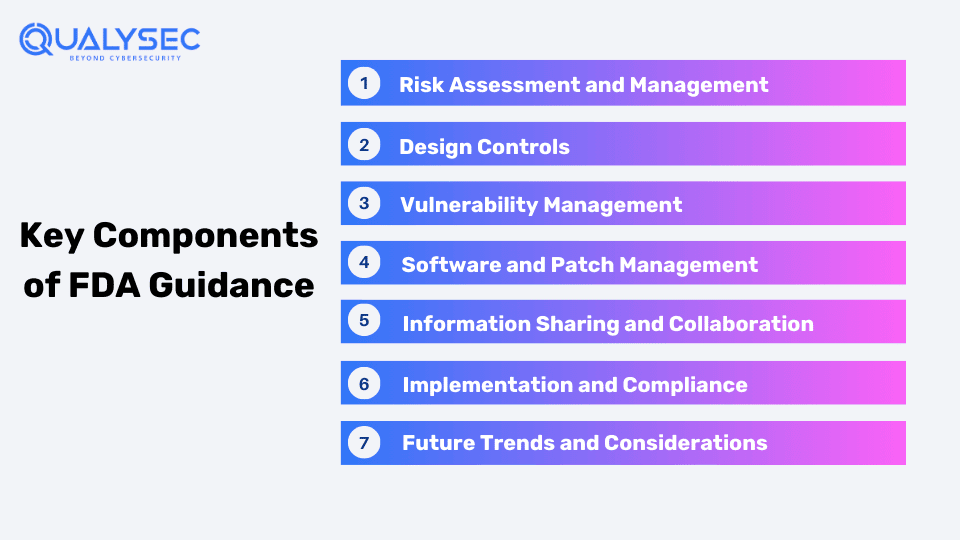
1. Risk Assessment and Management
The FDA’s statement regarding proactive cybersecurity risk assessment highlights the criticality of protecting medical devices from present as well as future threats. Integration of risk management right into the design and development process will enable manufacturers to find and fix vulnerabilities before they become major issues. This method not only boosts device security but also encourages people’s confidence that the technology is safe and reliable. Furthermore, with the help of the broad risk assessment strategy, manufacturers can tackle cybersecurity in order. To make sure all devices can overcome cyber threats at any given time during their whole lifetime.
2. Design Controls
FDA regulations make a precondition for developers of medical devices to implement design controls and validation in detail including FDA cybersecurity in medical devices. These standards form the base of pillars that ensure that the devices meet the stringent safety and efficacy criteria. Through adopting strong design controls, manufacturers can systematically manage product development in all phases, beginning from the initial product idea until it is launched. Thus, ensuring the device can adequately and safely perform the clinical functions intended for it.
Evaluation and validation techniques ensure that the controls are effective in the continuous verification of the performance of the devices. Within the parameter specifications, the risks are reduced and the patient outcomes remain improved. Additionally, this system setting not only creates product safety regulations but also creates a chance for innovations and continuous process improvements.
3. Vulnerability Management
Vulnerability Management is a systematic process that involves the detection, assessment, and mitigation of potential system weaknesses in infrastructure, software, or procedures. Organizations should remain alert and responsive to their possible risks, by taking a proactive approach to the identification and remediation of security loopholes before their exploitation by cyber-criminals. Therefore, this mechanism plays an important role in ensuring that there are no security breaches, data breaches, and other incidents that could lead to the loss of sensitive information or breakdown of operations.
Want to secure your business from cyber threats? Qualysec Technologies provides process-based vulnerability assessment and penetration testing (VAPT) services for web apps, mobile apps, networks, cloud, APIs, IoT devices, and more. Click below to fix an appointment!
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
4. Software and Patch Management:
Software and Patch Management are vital, especially in such industries, where software integration in medical devices and pharmaceutical processes is present. Keeping software systems stable and secure through regular patching, updates, and other procedures is a vital requirement. It ensures the system’s performance and compliance with industry regulations. Hence, with rapid response to vulnerabilities and meeting the standards set by the regulatory authorities, organizations can reduce risks that their systems and processes may experience due to software vulnerabilities.
5. Information Sharing and Collaboration:
Coordination and communication among stakeholders is paramount to ensure the security and efficiency of the health products including FDA guidance for medical device security. The collaboration of manufacturers, regulators, healthcare providers, and patients in the exchange of necessary information concerning the development, testing, side effects, and patient information is a must. Therefore, through this collaboration, a thorough comprehension of the product life cycle has been achieved. Further, it enables the organization to respond quickly to market trends and improve the quality of products, initiate the production of better products, and ensure the safety of patients.
6. Implementation and Compliance:
The management of regulations and standards in organizations is fundamental to the prevention of accidents and the improvement of product quality. It is continuous compliance that safeguards manufacturing processes, distribution channels, and healthcare practices from risks. It therefore ensures of good reputation and the approval of the authorities. Organizations should establish well-governed systems for compliance monitoring and enforcement. Additionally, includes periodic audits and quality control measures that can quickly detect and correct any deviations.
7. Future Trends and Considerations:
Keeping track of future trends, technological advancement, regulatory change, and emerging risks is fundamental for remaining competitive and efficient in the medical products industry. Firms should consider conducting research and development, ready to face changes in consumer preferences, as they should continually update their strategies as well. Additionally, by planning proactively, organizations can benefit from opportunities. Thereby, they can reduce threats, protect the organization against setbacks, and remain constantly relevant and open to success in a dynamic market environment.
Conclusion
Maintaining compliance with FDA guidance for medical device security becomes the key aspect as the medical device security landscape is in constant change. These regulations in addition assure that devices are secure and maintain trust between patients and healthcare providers. Embracing active risk assessments, strong design controls, and effective collaborations are the keys to successful cybersecurity strategies in healthcare manufacturing. Consequently, focusing on security mechanisms provides not only certainty and functionality to patients’ data but also maintains the integrity and efficacy of medical devices in the world of connected devices.
Frequently Asked Questions:
Q. What is the role of the FDA in medical device cybersecurity?
A. The FDA is a key player in medical device cybersecurity. It is responsible for the regulation and control of the cyber security of medical devices. Moreover, this covers evaluating cybersecurity risks, publishing guidelines, promoting industry standards, and assessing the efficacy and safety of these devices.
Q. What is the safety standard for medical devices?
A. The safety standard for medical devices is based on regulations like ISO 13485, FDA 510k Submission Guidance, and US FDA Guidelines. Additionally, it embraces vigorous testing, quality management systems, risk assessment, and conformity to certain criteria to guarantee the safety of the patients and the product’s efficacy.
Q. What is the security testing for medical devices?
A. Security testing for a medical device includes discovering any possible risks in the hardware, software, and network connection. Furthermore, it involves penetration testing, code review, risk analysis, and compliance with FDA pre-market guidance as a part of patient safety and data protection measures.
Ensure your healthcare solution is globally compliant.
Qualysec helps you meet HIPAA, FDA, ISO, and more. Contact us today!

Ensure your healthcare solution is globally compliant.
Qualysec helps you meet HIPAA, FDA, ISO, and more. Contact us today!




















































































































































































































































































0 Comments