Bringing an innovative medical device to the market demands more than modern technology. The U.S. Food and Drug Administration (FDA) has established strict guidelines to make sure that medical devices are safe from cyber threats. Meeting stringent FDA cybersecurity requirements is a difficult milestone for health tech startups and IT security professionals. A significant and often overlooked piece of this puzzle is penetration testing.
Penetration testing is more than a box to check; It is an important process that validates a medical device’s ability to withstand cyber threats. FDA cybersecurity regulations increasing focus on cybersecurity for both premarket and postmarket submissions, choosing the right penetration testing partner can make a big difference. But how do you decide whom to trust with such an important task? This blog will guide you on this.
Understanding FDA Cybersecurity Requirements
Before selecting a testing partner, it is necessary to understand the FDA cybersecurity expectations. Their guidelines are designed to protect patient safety and data integrity.
Key Guidelines
The FDA mandates that devices must be designed and maintained with a lifecycle approach to cybersecurity. This includes processes to assess, monitor, and address vulnerabilities. This means demonstrating that your device can handle realistic cyber threats for both premarket and postmarket submissions.
FDA cybersecurity guidance also emphasises the importance of risk mitigation. Manufacturers must provide detailed evidence of their efforts to secure devices against unauthorized access, data breaches, and other malicious activities.
The Role of Penetration Testing
Penetration testing is a hands-on, simulated attack performed to uncover vulnerabilities in software, hardware, or system architecture. For FDA submissions, this type of testing supports both premarket requirements, by showing thorough testing during design and postmarket requirements, by monitoring and maintaining security throughout the product lifecycle.
In simple words, penetration testing is your best partner that ensures the safety and effectiveness of your device.
Why Choosing the Right Pentesting Partner is Important?
Regarding penetration testing, not all testing partners can handle the unique challenges of FDA medical devices. The right choice matters because:
The Stakes of Getting It Wrong
Failure to demonstrate cybersecurity resilience can lead to your device being denied FDA approval. Such a setback delays time-to-market and could risk your company’s reputation and investor confidence.
Beyond approval delays, inadequate penetration testing increases the risk of vulnerabilities being exploited once the device is used. This can result in costly recalls, non-compliance fines, and, most importantly, patient safety risks.
The Expertise Gap
FDA guidelines are specific and challenging to meet without expertise in medical device security. Any regular testing company may lack the detailed understanding required for FDA guidance on cybersecurity assessments. This is why selecting a specialist with experience in medical device security is paramount.
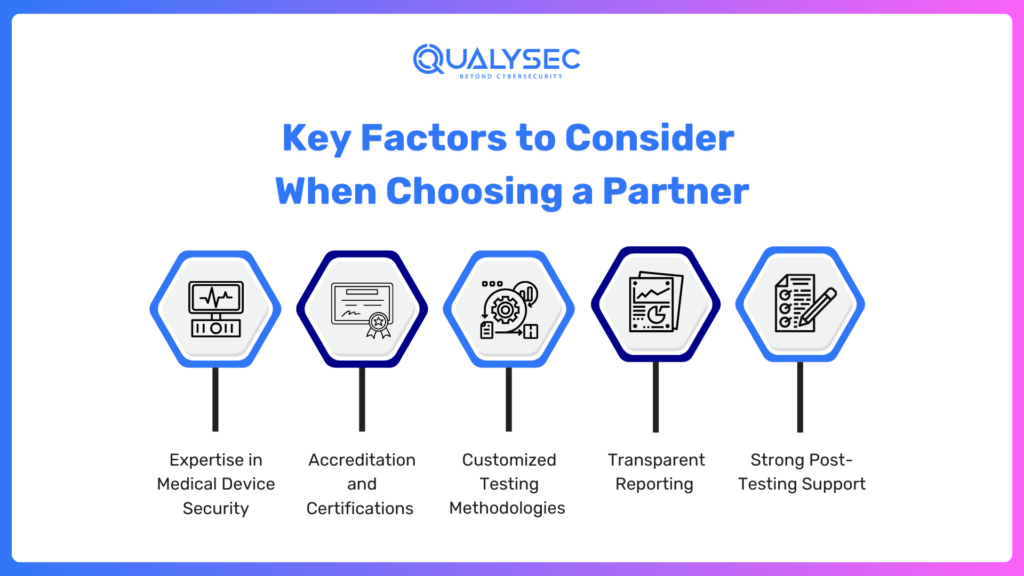
Key Factors to Consider When Choosing a Partner
When evaluating potential penetration testing providers, look for these essential features:
- Expertise in Medical Device Security
Choose a provider with a track record of passing through the unique cybersecurity requirements for FDA submissions. Ask for case studies or client references to ensure the provider knows the complexities of medical device architecture and software ecosystems.
- Accreditation and Certifications
Look for certifications like Certified Information Systems Security Professional (CISSP), Offensive Security Certified Professional (OSCP), or Certified Ethical Hacker (CEH). Make sure the provider adheres to standards such as ISO/IEC 27001. This demonstrates their commitment to rigorous security practices that align with FDA expectations.
- Customized Testing Methodologies
Medical devices vary greatly in design, functionality, and risk profile. A “one-size-fits-all” approach to penetration testing is ineffective. Your provider should offer a customized strategy based on the device type, software ecosystem, and potential threat model. The testing process must address application security, network vulnerabilities, firmware issues, and potential physical device exploits.
- Transparent Reporting
Thorough and precise reporting is critical for FDA submissions. Your partner should provide reports that outline all discovered vulnerabilities, their severity, and actionable recommendations for remediation. They should deliver reports in a format understandable to cybersecurity professionals and regulators during submission.
- Strong Post-Testing Support
Finding vulnerabilities isn’t enough. Addressing and documenting them for FDA compliance is equally important. Your testing partner should assist with fixing identified vulnerabilities and making sure your device is submission-ready. The partner should be available for follow-up testing or to assist with any additional documentation needed during the FDA review process.
Latest Penetration Testing Report

Red Flags to Avoid
Beware of these warning signs when selecting a testing partner.
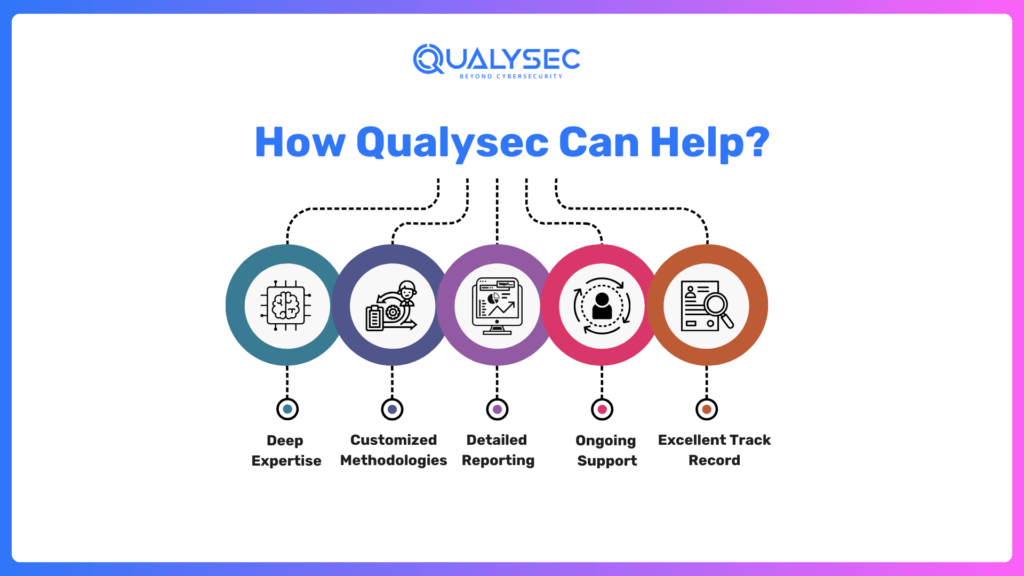
- Deep Expertise: Our team understands all particulars of medical device security and FDA standards.
- Customized Methodologies: We build custom testing strategies to fit the unique needs of your device, which covers all potential vulnerabilities.
- Detailed Reporting: Our reports make the FDA submission process seamless, from clear documentation to actionable recommendations.
- Ongoing Support: We don’t just find vulnerabilities; we help you address them so that you get all set for achieving compliance and readiness for any follow-up submissions.
- Excellent Track Record: Our proven track record speaks for itself, with countless satisfied clients who have successfully navigated FDA cybersecurity requirements.
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
Gain Regulatory Confidence Through the Right Partner!
Cybersecurity is no longer a secondary concern but a regulatory necessity seeking FDA medical device security approval. By choosing the right penetration testing partner, you ensure that you achieve compliance and attain device safety, patient trust, and operational success.
Don’t leave your FDA submission to chance. Partner with Qualysec for a thorough, transparent, and results-driven approach to penetration testing.
Contact Us Today for Your FDA-Compliance Testing Needs!
- Lack of Medical Device Experience: Avoid providers without proven medical device expertise or FDA submissions.
- Generic Methodologies: Steer clear of those offering cookie-cutter testing without customization.
- Poor Communication: Delayed or unclear feedback can disrupt your timeline and submission quality.
- Hidden Costs: Make sure pricing is transparent to prevent unexpected charges.












































































































































































































































































































































































































































0 Comments