Navigating the complicated regulatory environment surrounding medical devices might be difficult, particularly for technicians and executives who are not aware of the FDA’s standards and cybersecurity guidelines. Cybersecurity in FDA 510(k) Submissions is an essential part of introducing a medical product to the industry.
This thorough guide explains the importance of 510(k) in FDA cybersecurity entry, eligibility requirements, filing formats, and entry procedure, and provides helpful advice regarding an effective submission. We will also examine the eSTAR procedure for 510(k) uses, emphasising how it improves efficiency.
This thorough guide to completing the medical device cybersecurity FDA attempts to simplify the demands and offers many useful and realistic advice that one can start using right away. This blog aims to support you in reducing your journey to the marketplace by giving you detailed instructions on how to submit a 510(k) to the FDA.
What is 510(k)?
The FDA 510 k cybersecurity, a premarket filing provided by the FDA, is an essential phase in demonstrating a brand-new healthcare product’s significant equivalency to a lawfully commercialised reference technology. The filing is also essential to achieving approval to authorise the device’s U.S. advertising.
Qualifications for 510(k) Clearance
A device used for healthcare purposes needs to be substantially the same as a previously already-approved product in order to qualify for a 510(k) application. Comparisons in achievement, technical characteristics, and ultimate usage are all part of the analysis.
The Three Different Kinds of 510(k) Submissions
- The Classic 510(k)
This kind of proposal is particularly thorough and appropriate for equipment with notable technological advancements or others lacking a legitimately advertised baseline.
- The 510(k) designation
Useful whenever the equipment satisfies mandated requirements, making the proof of meaningful equivalency easier.
- The Unique 510(k)
This is intended for alterations to current gadgets, with an emphasis on proving that the adjustments have no impact on performance or security.
The FDA’s Submissions Procedure for 510(k)
- Locate The Predicate Gadget
Find a comparable predicate gadget which has been lawfully advertised. To prove considerable equivalency, this contrast is essential.
- Construct a Framework for Quality Control.
Implement a strong quality management system (QMS) to guarantee constant high-quality goods and adherence to FDA cybersecurity guidelines.
- Equipment Evaluation
Perform appropriate evaluations and research to ensure the device’s security and effectiveness. This comprises survival testing, technology confirmation, and other applicable examinations.
- The Submission
Create and submit the 510(k) implementation, which should include complete details about the gadget, its similarities with the qualifying apparatus, and any additional documents.
Five Pointers to Support The FDA 510(k) Application
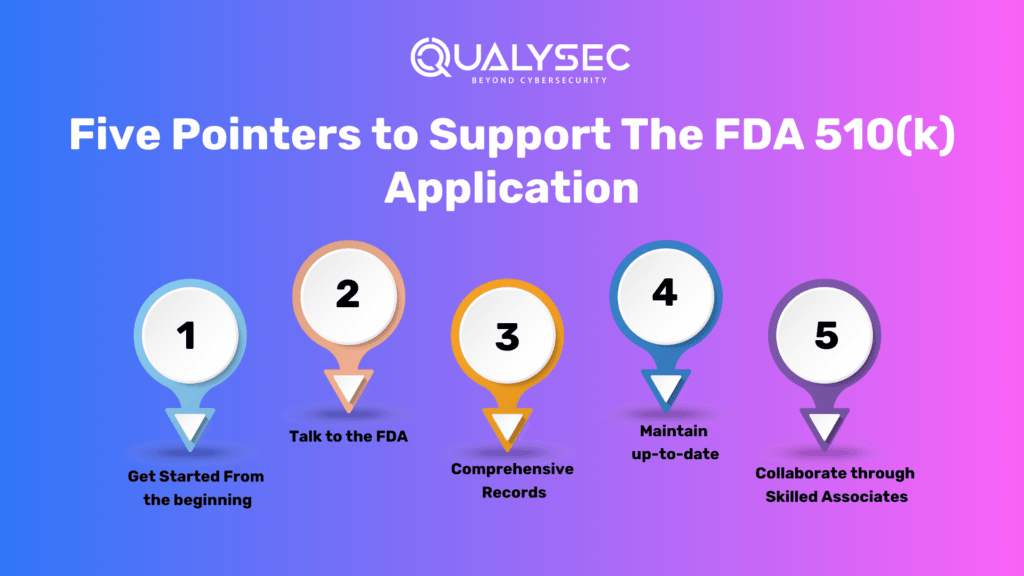
- Get Started From the Beginning:
To enable comprehensive evaluation, paperwork, and possible changes, start the planning procedure thoroughly in time.
- Talk to the FDA:
Immediately during the entire process, contact the FDA for advice, address any questions, and guarantee a more seamless application.
- Comprehensive Records:
Give extensive and understandable paperwork that includes procedures for testing, findings, and an extensive contrast between the reference items.
- Maintain up-to-date:
During the application method, stay up to date on FDA recommendations, laws, and modifications that could impact it.
- Collaborate through Skilled Associates:
Engage in consultancies or law enforcement specialists who are familiar with FDA applications to guarantee compliance.
Information on the Food and Drug Administration’s guidelines about healthcare devices
Companies can use a variety of methods and instruments to remain aware of modifications to rules that affect FDA medical device cybersecurity. Following are a few efficient methods for getting modifications:
- Acknowledging the FDA website
- FDA mail subscription
- FDA guidelines for documentation
- Federal Registration Act
- Market associates and analysts
- Webinars and conferences
- Compliance news sites
- FDA customers meet
- Consulting service
- Networking with compliance professionals
- Well-known publication houses and journals
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
Utilize contemporary methods such as Matrix and collaborate with seasoned allies. 510(k) criteria by the FDA Submission
Constructing Conditions provides an extensive solution for the cybersecurity guidance FDA procedure. MatrixRequirements is an online environment that enables the establishment of quality control processes and improves the total effectiveness regarding the procedure for submitting requirements.
Their toolkit enables the compilation of extensive documentation on technical subjects, risk evaluation, and verification, guaranteeing FDA certification.
Companies may use the Matrix Requirement solution to utilize documents and data to enable compliance with MDR and FDA regulations. Compliance professionals are assisted in creating the application material for FDA approval by designating files and data to be used in accordance with different requirements.
However, because the the aim of application form is constantly changing, a user experience is still in its infancy, therefore it will continue to be completed and finished out by hand.
Ensure your healthcare solution is globally compliant.
Qualysec helps you meet HIPAA, FDA, ISO, and more. Contact us today!























![Top 20 Network Security Companies in USA [2025 Updated List]](https://qualysec.com/wp-content/uploads/2025/05/Top-20-Network-Security-Companies-in-USA-2025-Updated-List-scaled.jpg)










































































































































































































































































































































































































0 Comments