It makes no sense to have an approach to risk management in medical devices that would make them effective, yet not safe for use by humans. Therefore, designing and developing medical devices will have to always run the regulatory gauntlet of FDA and ISO quality systems regulations with risk-free devices.
What is the process of risk management?
- Identify dangers: Generally, finding all possible hazards in the design of the device has been completed, such as flaws within the design, and defects in the software and manufacturing.
- Analyze all relevant risks: Assess all likelihoods and impacts of the potential risks.
- Prioritise risks: Those would be created by and possible areas that should promote immediate attention.
- Control risks: Reduce, mitigate, or eliminate risks through overt actions and measures.
- Monitor risks: Monitoring the effectiveness of the measures taken to control the risks.
Security Management and its Function in Medical Devices
Risk management medical device product development lifecycle is an integrated part. It assures the reliability of the product, performance as expected, and no harm to patients, operators, and the environment. Hence, in summary, risk management is a means to reduce or mitigate the chances of failure of the product.
ISO 14971:2007 specifies and describes the procedure related to possible hazards concerning the risk assessment medical device in concern, which must be followed by (and is, in fact, a must for) the manufacturers of medical devices.
In something very similar to ISO 14971, there are many other regulations relevant to risk management steps in the development of medical devices. These may approach risk management in different directions, but the end objective is the same.
Procedures for Medical Device Risk Management
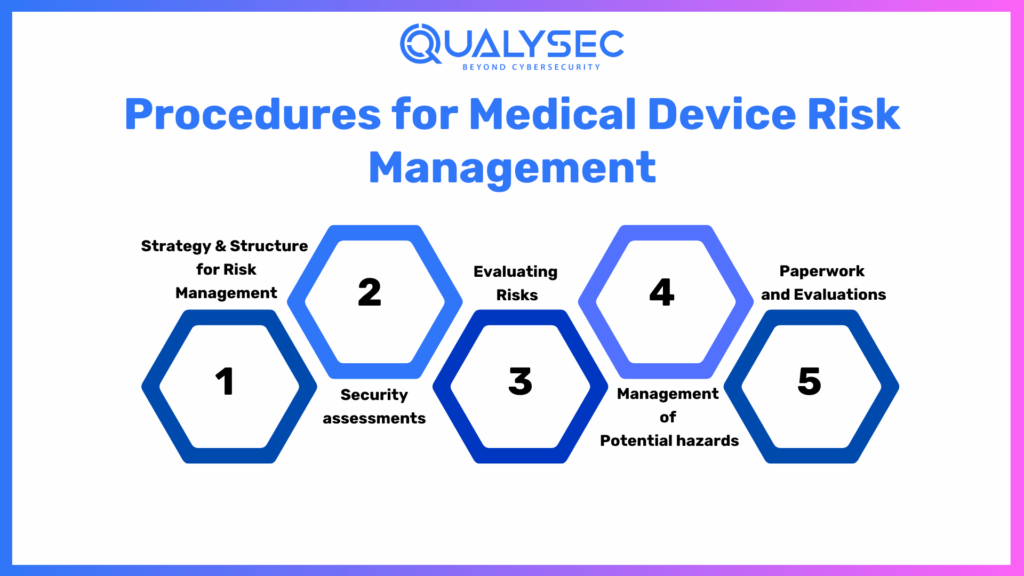
Commonly employed in the assessment of numerous procedures, systems, and practices for analyzing, evaluating, managing, and monitoring risks, medical devices fully adhere to managing these aspects effectively. Let’s look at the standard steps in preparing an all-inclusive lifecycle for medical device risk management.
Strategy & Structure for Risk Management
Application of any risk management process per the relevant regulations such as FDA or ISO needs to be supported by a risk management framework.
This framework encompasses the procedures of the actual development of the device and the definitions of roles and responsibilities for people associated with the device development project.
Furthermore, proper documentation of the risk management plan is also a requirement to be incorporated into the risk management framework for medical devices.
Security assessments
This phase of risk analysis will guide the manufacturers towards employing security risk management in determining the product’s intended use, thereby creating emphasis for the technical approach focusing on the relevant hazards (potential sources of harm).
During this phase, the standpoint of foreseeable hazards must be used in the earliest possible stage for risk assessment.
It is interesting in this context that, in risk assessment not for bearing on those causes only but also on certain potential risks associated with them.
Latest Penetration Testing Report

Evaluating Risks
Risk evaluation and quantification will be aided by determining the hazards’ frequency (likelihood) and intensity. In instances when one scenario is highly likely to take place but has minimal potential for destruction, and a different scenario has a high potential for adverse effects, it is advisable to properly visualize the danger on an array to determine whichever risk should be addressed initially.
Management of Potential hazards
Following identifying hazards, and managing them is the following stage, during which risk reduction is put into practice. Reducing the degree of threat to a manageable amount is the goal of managing risks.
Paperwork and Evaluations
Documenting the hazard control method and program is the final and most crucial stage. it’s also critical to remember that the threat control strategy need not only be documented in its early phases.
All of the behavior, states, evaluations, and illustrations produced for the duration of the risk management strategy phase must be included in the hazard administration record.
As risk management in healthcare plans integrates itself into the entire product development lifecycle processes, it is apparent that the documentation will remain active even beyond the end of product development activity.
Additionally, the successful implementation of control actions would also need to be documented along with the new risks that might arise as a result of the risk control action.
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
Conclusion
The processes detailed above were essential to completing the creation cycle of a healthcare innovation. The development of something that complies with the anticipated quality and security requirements is aided by establishing conformity via sufficient assessment of the threat control process. Risk management in medical devices is important for safety, compliance, and effectiveness and lastly for protecting patients and healthcare providers.






















![Top 20 Network Security Companies in USA [2025 Updated List]](https://qualysec.com/wp-content/uploads/2025/05/Top-20-Network-Security-Companies-in-USA-2025-Updated-List-scaled.jpg)










































































































































































































































































































































































































0 Comments