In a fast-moving global economy where FDA-regulated products, including medical devices, drugs, foods, and cosmetics, are brought to the U.S., there are strict rules enacted by the FDA that require location compliance. For foreign companies, appointing an FDA Agent (known as a U.S. Agent) in the U.S. can be particularly important.
The FDA Agent, either a person or a firm, is located in the U.S. and serves as the communication link between the FDA and foreign manufacturers. As of mid-2025, the FDA continues to enforce 21 CFR § 207.69 with the importance of knowing who the U.S. Agent is clear.
Why the fuss? Why all the concern? A credible U.S. Agent ensures you will receive urgent notifications, timely inspections, and avoid fines. In a world driven by transactions and emails, missing a regulatory request could jeopardise your product launch, especially given the FDA’s current response windows for regulatory requests, which are as short as 10 business days.
In this post, we’ll unpack everything from understanding what an FDA Agent does to selecting an FDA Agent, and what Qualysec can do to help you meet this requirement.
What Is an FDA Agent?
An FDA Agent, also commonly referred to as a U.S. Agent, is an individual or organisation in the United States designated by a foreign company to communicate with the FDA Regulations (21 CFR § 207.69) require each foreign establishment to designate a U.S.
Agent during the FDA facility registration. The U.S. Agent cannot be a P.O. box or an answering service – it must be a physical address with someone available during normal business hours.
Their role? It’s simple and important: to ensure all FDA communication gets to the foreign manufacturer, including invites to inspections, holds on import, emergency alerts, etc. Thus, the U.S. Agent is responsible for accepting those alerts and routing them as needed. The FDA will use the U.S. Agent if it cannot reach the manufacturer directly; therefore, the U.S. Agent has to respond in a timely fashion.
And they still have to take this very seriously – in 2025, the FDA still strictly enforces this requirement as we sit here today, and if you’re late or do not confirm within 10 days of designation (there are “Action Required” emails for the U.S. Agent to open, deal with and confirm) the registration gets cancelled.
Role and Responsibilities of a US FDA Agent
Your U.S. Agent is not an intrusive watchdog—they are your direct communication connection. For example, U.S. Agents are responsible for:
- Receiving, routing, and replying to FDA communications (including emergencies).
- Helping facilitate and coordinate FDA inspections at the foreign facility.
- Answering FDA inquiries regarding imported products.
- Acting as the FDA’s fallback communication contact if direct communications are impossible.
This has real-world implications. For instance, if an FDA import hold or a recall alert happens, your U.S. Agent is the one who ensures that the communication happens in a timely way. They do not act as an intermediary for adverse event reporting, nor are they required to submit 510(k)s, as that is the role of the manufacturer.
In 2025, the FDA is firm that U.S. Agents are available by telephone during business hours—no exceptions. U.S. Agents process urgent communications, assist with scheduling, and keep foreign companies compliant with the FDA.
Trusted FDA Agent services backed by cybersecurity expertise—schedule a call.
Who Needs to Appoint an FDA Agent?
If you are a U.S. entity, you don’t need a U.S. Agent — you are already local. If you are a foreign-based company manufacturing or exporting FDA-regulated products (medical devices, foods, cosmetics, or pharmaceuticals), you must select a U.S. Agent.
This applies to:
- Foreign manufacturers of devices, contract manufacturers, and sterilisers.
- Foreign food facility operators.
- Foreign drug companies, IND/NDA/ANDA applicants.
- Even foreign cosmetic companies are under MoCRA.
Why? Without a U.S. Agent, your FDA establishment registration will not be completed, and your products may be refused entry or seized at the port of entry. As of March 2025, regulatory advisors (e.g., Artixio) point out that all entities, from “primary manufacturers” to specification developers, will require a U.S. Agent.
FDA Agent Requirements
What does the FDA look for in a U.S. Agent? Legally, they must:
- Reside or maintain a business in the U.S.—a real office, not just a mailbox.
- Be available during standard business hours by phone
- Confirm their role via an “Action Required” email within 10 business days—if not, the foreign firm must reassign.
- Handle FDA communications and inspection coordination, but they don’t need to submit adverse events or 510(k).
From a practical standpoint, agents often also:
- Help maintain accurate FDA registration info.
- Prepare and forward emergency or deficiency notices (e.g., labeller code issues).
- Assist with scheduling FDA inspections, helping manufacturers prep ahead of time.
As of 2025 rules, each foreign facility gets only one U.S. Agent, unless multiple establishments are registered separately.
Why Having a Reliable FDA Agent Matters
Empowering your U.S. Agent isn’t just a checkbox to tick; it keeps your business moving. If you’re exporting life-critical products (like medical devices), nothing is more important than the timeliness of communications with the FDA. If the FDA identifies a cybersecurity issue or any other reason to flag your import, your agent ensures FDA communications reach your business to the appropriate people and as quickly as possible.
Your proactive agent will help provide clarity while wading through the regulatory waters. As the firm Qserve outlines, agents can serve as full-service liaisons by providing not only the FDA’s contact information but also helping with inspection preparations, device listings, regulatory questions, and anything else you may need.
Your agent will prove useful as they are vital in emergencies. The FDA’s guidance on agent roles indicates that your agent must perform “reviewing, routing, responding to all communications, including emergency communications”. That’s not a simple task; one missed call or one email not being forwarded could lead to regulatory compliance actions or issues with product refusals.
Experts also say that it’s critical to respond quickly; sometimes the FDA expects you to respond within a week or less. Missing that may push off the approval or shipment of products to customers, which could cost you thousands.
FDA Agent Vs. FDA ASMR Consultant: What’s The Difference?
You may have seen ‘FDA ASMR Consultant’ circulate—that is a marketing term, not a regulatory term. Although both help clients with FDA matters, here are two forms of engagement that are not aligned:
FDA Agent (U.S. Agent): A legal regulatory requirement under CFR 207.69. The legal Agent is responsible for communications with the FDA, and inspection, as well as the obligation to receive FDA official notices.
FDA ASMR Consultant: A non‑regulated service that positions the offering to be in line with ‘American Sensory and Medical Regulation’ type of consulting, which is often consulting on technology or messaging, inspection preparedness, clinical, quality systems, or cybersecurity. This role is not a legal substitute for a U.S. Agent.
Essentially, The Agent’s role is to aid the company in meeting compliance standards. The ASMR Consultant will work with the company to optimise their time to compliance. As a recap, you still need a registered U.S. Agent.
How to Choose the Right FDA US Agent
Picking the best U.S. Agent in 2025 means going beyond a cheap address. Here’s what to look for:
- FDA Regulatory Experience – Choose someone who knows 21 CFR 207, FURLS registration, import holds, cybersecurity trends, and can guide you accordingly.
- Reliability & Availability – They should pick up phone calls, respond to urgent notices fast (within seven days or less).
- Communication Channels – Good agents keep records of notifications and updates in writing.
- Value‑added services – Does the agent offer mock inspection prep, audit support, FURLS updates, or advice during submissions? These extras help you stay ready.
- Transparency – Avoid hidden‑cost agents. Some charge low annual fees (e.g., $250–$300/year) for basic service; extras cost more.
In 2025, it pays to review recent agents’ case studies—for instance, Qualysec emphasises regulatory guidance in cybersecurity, while Qserve offers a combined agent and correspondent package.
How Qualysec Helps You Meet FDA Agent Requirements
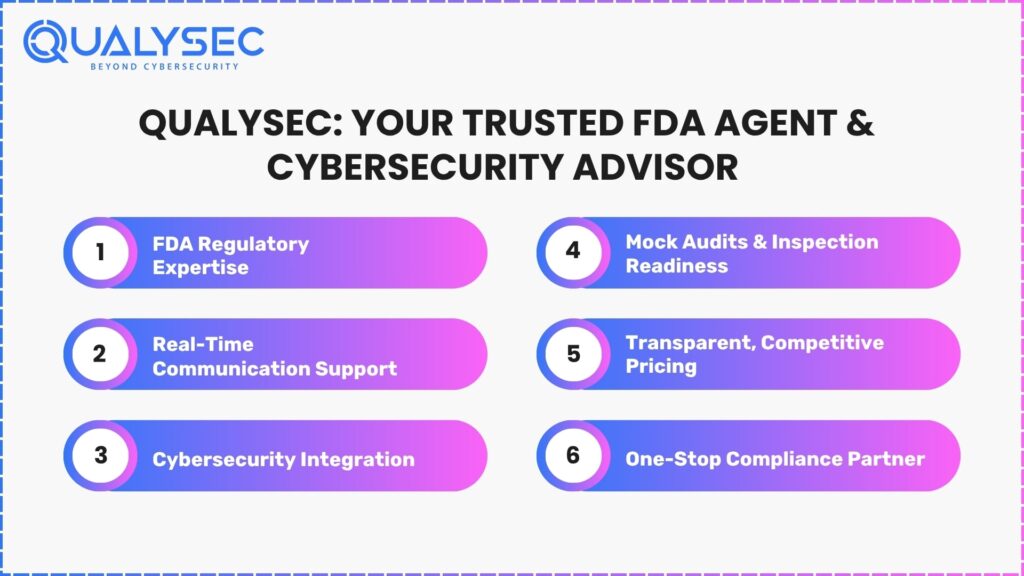
Qualysec is primarily known as an FDA cybersecurity consulting organisation; however, they also assist in fulfilling obligations as a FDA Agent by acting as your Team Services and Advisory partner. Here’s how they can assist you:
- Regulatory Knowledge: The Qualysec team is familiar with current FDA guidance documents, including the 2025 update on Medical Device Cybersecurity as part of the 510(k) or PMA content.
- Direct Communication: They can help draft practical responses and prepare you for phone conversations, inspections, or urgent notices.
- Inspection Preparation: Qualysec can offer mock audits and assist you in preparing your facility for an FDA inspection to meet the FDA’s criteria.
- Cybersecurity Expertise: Qualysec’s expertise in Medical Device Pentesting and 510(k) Cybersecurity Dossiers puts you ahead of the game since Cybersecurity is now part of FDA compliance.
- Competitive and Transparent Pricing: With known financial costs and service bundles, they give clarity, no billing surprises on registration and renewal.
To summarise, Qualysec offers value, beyond labelling—when you hire them, they assist with a Company’s FDA Agent duties, while aiding you to meet modern expectations of Cybersecurity for your device.
Secure your FDA compliance today with Qualysec—book your free consultation now.
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
Conclusion
Selecting a trustworthy FDA Agent is not just a legal requirement—it is part of doing business in the United States. As a foreign manufacturer, having a reliable U.S. Agent is important for smooth communication with the FDA, avoiding unnecessary delays, and keeping your products moving through the supply chain. When selecting a U.S. Agent, choose one that has experience, is responsive, and remains current with recent FDA expectations. In 2025, compliance is the name of the game, and the right FDA agent can help you and your business.
Qualysec makes FDA communications seamless—talk to an expert today.
FAQ’s
1. What does an FDA agent do?
An FDA agent serves as a representative in the United States for foreign companies and handles all formal communications between the FDA and the manufacturer.
2. Is an FDA agent required?
Yes, if you are a foreign manufacturer of FDA-regulated products selling goods in the United States, you will need to select a U.S. FDA agent, and it is required by law.
3. What is the cost for an FDA US agent service?
Fees range from $250 to $750 annually, depending on what type of services the agent provides and the support level offered.

















































































































































































































































































































































































































































0 Comments