Managing the intricate process of medical device regulation in the U.S. is not always straightforward, particularly for engineers and managers who are new to the requirements of the FDA. One very important part of getting a medical device to market in the United States is the FDA 510k Submission process. This all-encompassing manual will shed light on the mystery of the 510(k) submission, including its importance, qualifications for acceptance, types of submissions, and submission procedure, and provide important advice on successful submission. We will examine the FDA 510k Submission and the benefits of how it promotes efficiency.
Eligibility Criteria for 510(k) Clearance
For eligibility for a 510(k) filing, a medical device must be substantially equivalent to a legally marketed device. The comparison includes similarities in intended use, technological features, and performance.
The Three Types of 510(k) Submission
The Traditional 510(k)
This is the most detailed form of submission, appropriate for devices with no legally marketed predicate or with substantial differences in technology.
The Abbreviated 510(k)
Useful when there are established standards to which the device is able to conform, making it easier to prove substantial equivalence.
The Special 510(k)
Intended for adjustments to already available devices to show that the adjustments do not influence safety and effectiveness.
“Explore our recent article: FDA Penetration Testing: Why It’s Vital for 510(k) Submission”
Latest Penetration Testing Report

The FDA 510k Submission Process
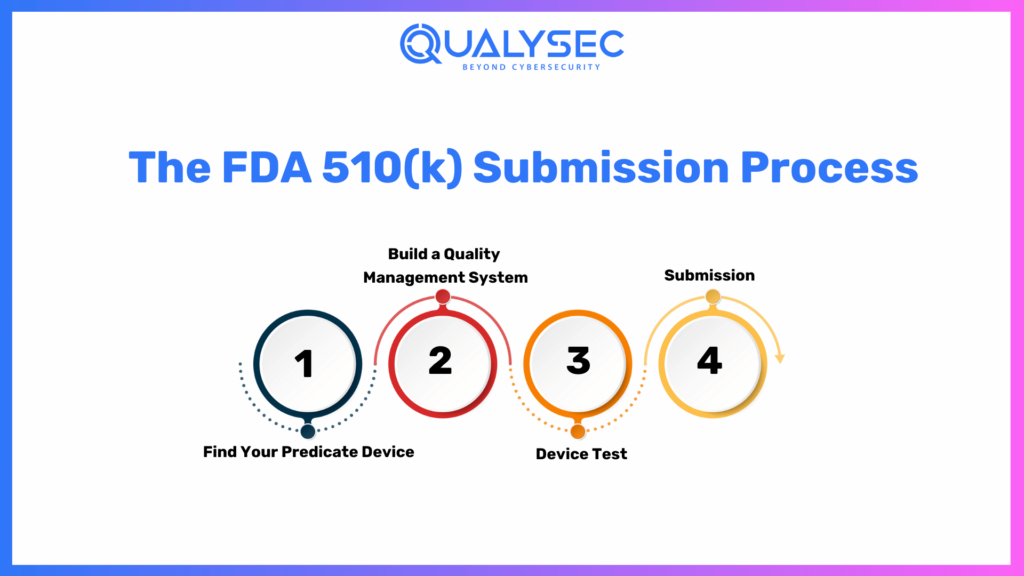
Find Your Predicate Device
Identify an FDA-marketed predicate device which is comparable to yours. Comparability is the key to illustrating substantial equivalence.
Build a Quality Management System
Develop a robust Quality Management System (QMS) to manage product quality with consistency and support FDA compliance.
Device Test
Perform needed tests and studies to establish your device’s safety and effectiveness. This involves biocompatibility, software verification, and other as-applicable testing.
Submission
Prepare and file your 510(k) application, along with in-depth details regarding your device, comparisons with the predicate device, and supportive documentation.
“Also check our recent guide to FDA Cybersecurity Guidelines for Medical Devices“
The 510(k) speciality – the “predicate device”
The key distinction between MDR and FDA is that in the 510(k) procedure, a “predicate device” is taken as a reference for the safety and performance of the new device. The “predicate device” is an existing device on the market, which has substantial similar technical and clinical similarities – the “substantial equivalence”. Therefore, the 510(k) procedure depends heavily on the demonstration and justification for the equivalence of the new device with that “predicate device”.
When there is an available predicate device for a 510(k) filing and the new device is reasonably equivalent to the predicate, then the amount of clinical evidence needed can depend upon the risk classification of the device. The risk classification (Class I, II, or III) determines how much clinical data are required. This is a general outline:
1. Class I Devices:
- Class I devices are low to moderate risk.
- Clinical data may not be necessary, and substantial equivalence may often be determined by non-clinical testing and prior literature.
2. Class II Devices:
- Class II devices are moderate-risk devices.
- Clinical data may be needed, but it is frequently restricted to non-pivotal studies or established clinical principles.
- The manufacturer may use data from the predicate device to substantiate the FDA 510k submission.
3. Class III Devices:
- Class III devices are high-risk devices.
- Clinical information is usually necessary to prove the safety and efficacy of the new device.
- The volume of clinical data may differ according to the innovativeness of the device, whether there are alternatives for the condition, and how risky use of the device is.
- Even when a predicate device is being used as the basis of comparison, producers are required to offer adequate data to prove the new device as safe and efficacious as the predicate. Providing comprehensive data concerning device features, design, test performance, as well as alterations made to the device, forms part of these requirements.
Start early with the preparation of the FDA submission – save time from leaps after the design phase
“Read Also: FDA 510(k) Compliance and Why It Matters for Medical Devices“
Effectively obtaining a 510(k) submission involves planning and compliance with regulations. Below are important points for manufacturers to consider from the outset:
Key Considerations for a Successful 510(k) Submission
1. Early Engagement with the FDA:
- Early communication with the FDA establishes a successful process via the introduction of your device with other regulatory requirements and guidelines with respect to submission processes.
- Pre-submission meetings are quite helpful to facilitate clarification or resolution of concerns before formal submission.
2. Thorough Understanding of Regulatory Requirements:
- Gain a thorough understanding of the FDA’s regulatory needs, including guidance documents applicable to your device type.
- Keep current with updates and regulatory changes that could affect the submission process.
3. Building a Quality Management System (QMS):
- Create a solid Quality Management System (QMS) to maintain consistent product quality and FDA regulatory compliance.
- adopt either FDA 820 or ISO 13485 as base requirements for your QMS
4. Identification of Predicate Device:
- Select a predicate device legally sold that is substantially equivalent to your device.
- A predicate device is important for the demonstration of substantial equivalence and needs to be adequately documented.
5. Clinical Data and Testing:
- Assess the requirement of clinical data against the risk class of your device.
- Perform testing and studies to substantiate the safety and functionality of your device as necessary.
- Ensure documentation of test protocols and following accepted standards.
- Consider what can be drawn from the predicate device.
- Clinical evidence should have been established with US patients!
6. Documentation Preparation:
- Begin preparing thorough documentation early on in the development process.
- Record design and development work, risk analysis, test results, and any changes to the device –> do not want to do it once that step is passed!
7. Risk Management and Mitigation:
- Implement an effective risk management process to detect, evaluate, and mitigate risks related to your device.
- Shows how risk mitigation strategies are incorporated into the manufacturing and design processes.
- If you possess software that is internet-connected, cyber risk analysis must be demonstrated to FDA as well
8. Realistic Timelines and Resources:
- Set realistic submission timelines, keeping in mind how long testing, documentation, and revisions might take.
- Provide adequate resources, both personnel and expertise, to assist in the submission process.
9. FDA eSubmitter and eSTAR:
- Get acquainted with FDA eSubmitter, an electronic submission tool, and use it to prepare your submission.
- Learn about the eSTAR (Electronic Submission Template and Resource) process to take advantage of a standardized template and electronic platform.
10. Stay Updated on FDA Guidance:
- Periodically review updates to FDA guidance documents applicable to your device.
- Participate in FDA workshops, webinars, or training sessions to be aware of best practices and expectations.
“You might like to explore: FDA 510(k) Cybersecurity Risks: Ensuring Safe and Secure Medical Devices“
Detailed documentation – however what needs to be in?
The 510(k) U.S. Food and Drug Administration (FDA) submission must be documented sufficiently to establish that the new medical device is substantially similar to a legally marketed predicate device. Submission documentation needs to be complete, logically constructed, and compliant with FDA regulations.
The following are important details that are generally needed in the filing documentation for a 510(k) application:
1. Cover Letter:
A cover letter accompanying the submission, consisting of the contact details of the applicant, a summary of a brief device description, and intended use.
2. Table of Contents:
An in-depth table of contents indicating the organizational structure and setup of the submission.
4. Indications for Use:
A succinct statement of the use intended by the device, incorporating indications for use and limitations where applicable.
4. Device Description:
Thorough description of the device, components, material, design specifications, and characteristics.
5. Substantial Equivalence Discussion
A detailed comparison of the new device with the predicate device regarding intended use, technological features, and performance.
6. Performance Data:
Data from testing to establish the safety and efficacy of the device, including biocompatibility test results, software verification results, and any applicable performance test results.
7. Labeling:
Samples of the proposed labelling, such as instructions for use, warnings, precautions, and other information presented to the end user.
8. Proposed Labeling for Use in the U.S.:
If the device is labelled differently for use in the U.S., give the suggested U.S.-specific labelling.
9. Summary of Biocompatibility Testing:
A summary of the biocompatibility testing data, test methods, results, and conclusions.
10. Software Information:
Details on the software elements of the device, including verification and validation activities, and conformity to relevant standards.
11. Sterilization and Shelf Life:
Information on the sterilization processes employed and details on the shelf life of the device.
12. Summary of Clinical Data:
Where relevant, a summary of clinical data substantiating the safety and performance of the device.
13. Comparative Performance Data
Comparative performance data shows that any variation between the new device and the predicate device does not impact safety and efficacy.
14. Device Specifications:
Detailed technical specifications of the device, including drawings, schematics, and manufacturing procedures.
15. Quality Management System (QMS):
Information about the manufacturer’s QMS, including how it maintains the quality and consistency of the device.
16. Financial Certification or Disclosure:
A financial disclosure or certification of any payments, fees, or compensation paid or offered to any individual in connection with the filing.
17. Declaration of Conformity:
A statement of conformity to any relevant accepted standards.
18. Additional Information:
Any other information requested by the FDA or required to substantiate the filing.
“Also Check: How much does FDA 510 K approval cost?“
Work with Experienced Partners like Qualysec for Smooth FDA 510(k) Submission
Qualysec has a complete solution to speed up the FDA 510(k) submission process. If you partner with Qualysec, your FDA 510k Submission process could be much smoother, more compliant, and safer. Qualysec provides penetration testing services to assess cyber vulnerabilities, which could ultimately increase the safety of your medical devices from cyber threats. Do not leave compliance to chance – work with experts who understand regulatory and cybersecurity requirements.
Contact us today and partner with Qualysec to make sure everything goes smoothly with proper submission to the FDA 510(k).
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
Ensure your healthcare solution is globally compliant.
Qualysec helps you meet HIPAA, FDA, ISO, and more. Contact us today!



















































































































































































































































































































































































































0 Comments