For every pharmaceutical or medical device business hoping to launch a product, receiving FDA approval for medical device is an essential first step. This manual offers a thorough rundown of the FDA approval procedure, along with crucial actions and advice to make the road to legal approval as smooth as possible.
What is FDA Approval?
A good or service that has received FDA approval has undergone extensive testing for reliability, efficacy, and quality in production. before medicinal treatments, biological substances, and healthcare equipment can be sold to the general population, this authorisation is required.
The good or service is put through a number of examinations and assessments during the procedure for approval, which frequently consists of several phases, such as clinical trials and preclinical studies. In order to ascertain whether the good satisfies the requirements for use by customers, each stage is crucial.
While research studies are carried out in stages involving human subjects in order to assess the drug’s effectiveness and monitor any side effects, preclinical trials usually comprise laboratories and animal investigations to examine the item’s risk characteristics.
Latest Penetration Testing Report

FDA Medical Device Approval Procedure
You must follow several important rules and specifications, complete the certification management, and follow a single of the approval routes to have the goods approved. There are five phases in the whole procedure of getting FDA approval for medical devices such as:
The product’s certification
Classifying a medical device correctly is the first step towards receiving FDA clearance. Since the manufacturing method varies based on the device’s categorisation this phase is very crucial. The probability that medical gadgets present determines their classification.
Prior to starting the growth manage, the device’s categorisation must be established. A device’s category may have an impact on how standards and checks are handled. It also establishes the rules the gadget needs to follow.
Making an initial version
The creation of an initial or initial iteration of the medical device is the following stage in the approval procedure. This an item is designed for utilisation in controlled environments by academics and is not meant for use by individuals.
This phase promotes identifying and mitigating an item’s latent dangers and offers essential details about its possible applications.
The Approval Process
The device’s application procedure, known as the third stage, also depends on the prior approval. The FDA created the three mentioned earlier regulation categories for medical devices according to hazard.
In this case, regulatory oversight grows along with the product category. Producers have a variety of ways to select from because there are quite a few different device classifications. The following are the main routes to medical equipment authorization:
510(k) Premarket Notice
The primary method for introducing a medical gadget is this one. Nearly all Class II products as well as certain devices in Class I need it. Delivering proof and documentation that a medical product is significantly more secure and efficient than the gadgets or devices that are now lawfully available on the marketplace is the aim of this submission.
In addition to comparing the two devices with regard to of design oversight procedures and validation tests, the records must demonstrate that the devices have identical purpose and technological specs.
Premarket certification
Class III devices as well as those from Class I and Class II that were unable to offer significant equivalency in the 510(k) procedure are covered by the PMA route. Since research studies and empirical evidence are needed to demonstrate the medical device’s safety and efficacy, this procedure is especially difficult.
Humanitarian Device Exemption (HDE)
The manufacturer needs to obtain a HUD exemption before submitting the HDE application. Under a HUD exemption, the FDA performs an important assessment after preparing a decision review to assess the completeness of the submitted application.
During this procedure, the agency makes sure that there are no alternative options for introducing such a HUD to the marketplace and confirms that no substitutes for it are available.
FDA Tracking Performance of Post-Market Devices
Following clearance, the FDA keeps an eye on the device’s efficiency to ensure it’s secure and efficient in light of any emerging safety issues. Throughout the United States, the FDA inspects manufacturing plants both formally and informally.
These inspections, which are meant to make sure that manufacturers are adhering to the best procedures, can be regular or brought on by particular problems. The Food and Drug Administration has the power to vacate the premises if requirements are not fulfilled.
What figure does it take to have a medical device approved by the FDA?
Assuming the candidate qualifies for a permission, the FDA requires payment of an expense to begin the procedure for approval for the majority of its submissions for medical devices assessment.
The FDA levies an Annually Setup Application Cost, a total of $5,672 for 2022, on companies that wish to introduce their medical products in the United States in addition to the submission fees.
Details about the recently implemented fees is available on the MDUFA user fee page, and the charges are published in the General Register for every financial year. It is important to note that small firms may be eligible for discounted costs.
What period does FDA approval for medical devices require?
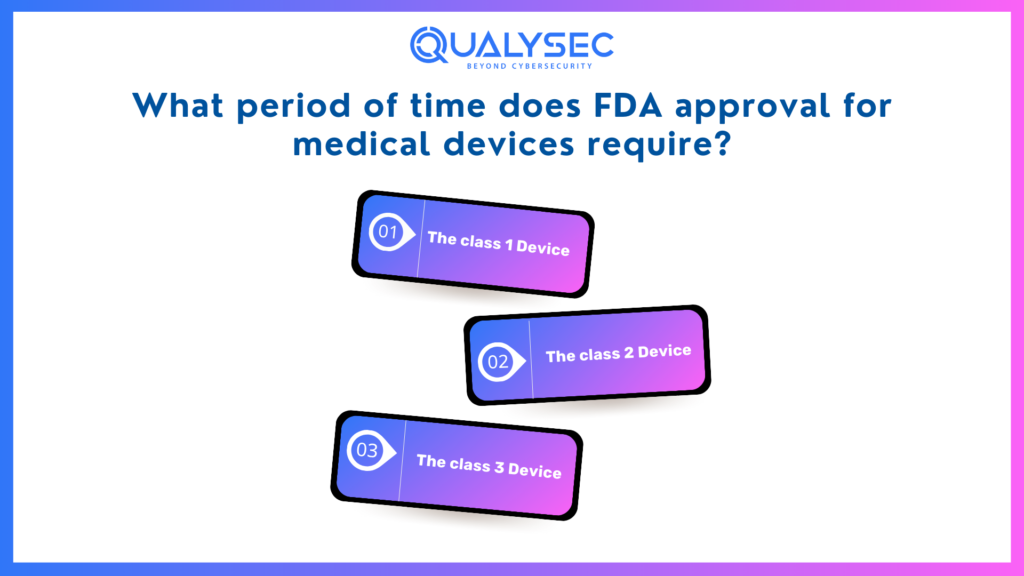
The duration required to obtain a medical device approved varies by class, between only a few days to a period of eight months.
The class 1 Device
Class I devices often have the quickest path to clearance. The vast majority of devices in Class I are self-registered and FDA-listed. The procedure consists of three steps:
completing the enrolment cost, uploading the application and membership information, and getting an acknowledgment electronic mail, all of which take around a week or so.
A limited fraction of Class I devices need 510(k) submission in order which is usually employed for Category II devices.
The class 2 Device
The vast majority of Class II devices, as well as some Class I devices, need a 510(k) implementation, making the procedure for getting approval longer.
If the use is accurate and there are no assessment concerns, the full procedure should take approximately 90 days to complete.
Yet, if there are any flaws with the procedure or deficiencies in the paperwork, the procedure for approval might take a lot longer.
The Class 3 Device
Devices classified as devices in class III frequently adhere to the PMA route, considered among the most complex and restrictive methods of registration. Initially, the Food and Drug Administration has 45 days to decide if the paperwork is adequately comprehensive and inform the person who submitted it of the outcome of the verification process.
The Food and Drug Administration now has 180 days to study the preliminary marketing authorization and decide whether to grant or deny the application. Again, if the Environmental Protection Agency detects errors or weaknesses in the request that must be addressed, or if the candidate provides new pertinent information or research, the procedure for approval may be doubled.
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
Conclusion
To sum up, getting FDA approval for medical device is a complex process that calls for meticulous preparation and compliance with rules. For every business hoping to sell products, getting FDA approval is essential, especially in the pharmaceutical and medical device industries.
Ensure your healthcare solution is globally compliant.
Qualysec helps you meet HIPAA, FDA, ISO, and more. Contact us today!


















































































































































































































































































0 Comments