Gaining FDA approval is important in this quickly evolving tech scenario. It is even more crucial for any pharmaceutical and medical-equipped organization to bring a product to the market.
This blog will provide complete knowledge on how to get FDA approval, including important procedures and recommendations to simplify.
What is FDA Approval?
FDA clearance indicates that a product went through rigorous testing for reliability, efficacy, and production quality. This authorization is required for pharmaceutical medications, biological materials, and medical devices before they may be sold to the general public.
The method of approval sometimes includes numerous stages, such as preliminary research and clinical investigations, during which the product is subjected to different testing and assessments. The entire process is crucial in establishing if the product satisfies the requirements for public usage.
Preclinical study trials often include lab and animal research to check the product’s security shape, whereas clinical tests are undertaken in stages with human volunteers that assess the drug’s efficacy and evaluate any side effects.
Understanding the FDA Approval?
The Food and Drug Administration is the organization in charge of assuring that food, beverages, pharmaceuticals, and healthcare products manufactured in the US are secure and efficient.
To do this, the Food and Drug Administration (FDA) demands that items designed for business purposes go through a comprehensive licensing procedure.
Why is FDA Approval Important?
FDA permission is necessary for a variety of purposes. Initially, it assures customers knowing the item in question has undergone proper examination and is certified secure for use. Furthermore, approval from regulators allows producers to obtain a presence in the marketplace, thereby boosting earnings and credibility for the brand.
Furthermore, FDA clearance can provide an edge over others by demonstrating that a firm conforms to the most stringent safety and quality requirements in the creation of its goods. It additionally decreases the likelihood of conflicts of law when the good is published.
The FDA’s thorough examination procedure not only safeguards customers, but it also promotes creativity in the industry by urging corporations to make investments in study and development.
This constantly changing environment results in the development of innovative medicines and gadgets that may significantly boost the lives of patients and enhance the standard of existence substantially many people.
Furthermore, the Food and Drug Administration continues to track goods long after they’ve been authorized. This afterward monitoring is critical for detecting any lasting consequences or unusual negative outcomes that might have been missed during research studies.
FDA Approval Preparation
Before beginning the FDA approval procedure, businesses must thoroughly plan to guarantee a seamless application. This preparedness can help avoid further postponements and issues.
Collecting Required Information
One of the first tasks in preparing is gathering the required documents. This involves medical research information, production methods, marking, and inspection and validation procedures.
Every bit of paperwork helps to back up the claim and show that the good fulfills FDA requirements.
Ensure that you comply with the Food and Drug Administration’s rules.
Compliance is an important part of obtaining FDA clearance. Businesses have to understand the precise limitations that are applicable to their product variety. This involves following Good Clinical Practice (GCP) principles and giving detailed security details.
Further, it is critical to establish a policy of compliance inside the firm. This includes teaching employees about laws and FDA regulations and developing a feeling of responsibility at all stages of the construction endeavor.
Ongoing internal audits and fake examinations are another way to detect possible violations before submission, enabling businesses to rectify concerns effectively and reinforce their whole entry plan.
The FDA Approval Procedure
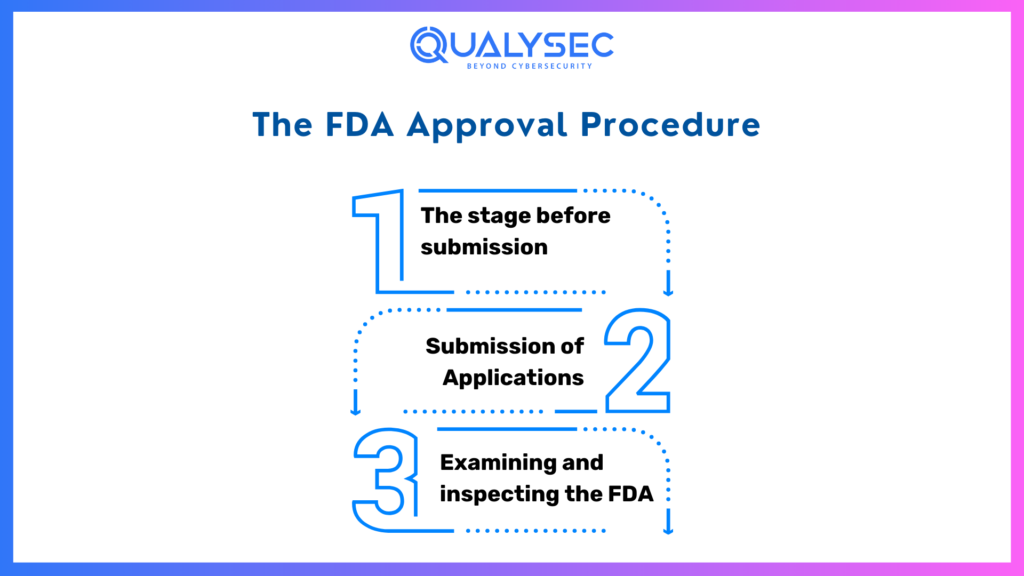
The FDA approval procedure is divided into numerous steps, each one carrying a unique set of standards and dates. Knowing this paradigm will assist businesses plan more effectively. These steps include:
- The stage before submission
- Submission of Applications
- Examining and inspecting the FDA
After The FDA Approval Phase
After receiving FDA clearance, firms have to keep adherence and audit their products to ensure their continuous quality and value by:
Keeping regulatory Compliance
Following clearance, producers must follow strict standards to maintain their items in the marketplace. This involves adhering to Current Good Manufacturing Practices (CGMP) and carrying out after-market research as required by the Food and Drug Administration (FDA).
Following Approval Tracking and Reporting
Businesses have to create mechanisms for continuous tracking and notification of any possible adverse effects or shortcomings in products to the FDA. This component is critical for sustaining safety for everyone and confidence.
Frequently planned assessments and feedback chains are critical components of this approach because they enable rapid changes and enhancements according to practical information and customer reviews.
Typical Difficulties in Getting FDA Verification and Approvals
Knowledge Regarding the Regulatory Language
The FDA’s regulation terminology may be complicated and frightening, which frequently leads to errors or misconceptions of standards. Businesses must make an effort to grasp these subtleties to assure adherence.
Partnering with commercial research organizations can assist in closing this gap in research. Their professionals advise on legal terminology, guaranteeing that businesses comprehend and carry out regulations effectively.
Effective Time and Resource Management
Obtaining FDA clearance is a laborious and costly procedure. Businesses must prepare accordingly to minimize disruptions that might result in additional expenditures. Working with a CRO can help you manage your resources more effectively.
Exporting specific tasks allows businesses to concentrate on important tasks and utilize their finances more wisely, resulting in an improved clearance procedure.
Conclusion
In conclusion, seeking FDA clearance is a comprehensive process that involves meticulous preparation and FDA compliance with laws. Partnering via a qualified business may provide considerable benefits, easing the procedure and increasing the chance of acceptance.
Are you eager to manage the FDA approval procedure with competence and effectiveness? Select a reliable organization for excellence that can provide a full range of CRO services to handle the entire trial from beginning to end. Don’t allow the drawbacks of approval by the FDA to drag your business down.




















































































































































































































































































0 Comments