The FDA Penetration Testing plays a crucial role in ensuring the safety and security of medical devices. This significance arises from the requirement for these devices to undergo evaluation and obtain clearance from the FDA before being sold in the United States. Much has been written about such processes; these would include the increasing focus on cybersecurity over recent years.
This feature will clarify several aspects of relevance concerning cybersecurity when one submits a 510(k) and PMA filings towards medical devices, with a further focus on cyber-medical approvals.
Let us delve deeper into FDA 510k submission and why regular FDA 510(k) vulnerability assessment is vital for cybersecurity.
What is FDA Penetration Testing?
FDA penetration testing is a comprehensive testing process during which simulated cyber attacks are undertaken by cybersecurity experts to locate flaws in medical devices and, very importantly, fix these problems. That way forward, the medical devices will be highly secured against known as well as unknown threats at every stage depending on the launching to their lifecycle.
Objectives of FDA Penetration Testing
- Identification of vulnerabilities: Find weaknesses in systems that would allow outside hacking to occur, then remediate these weaknesses.
- Ensure compliance: Federal regulations on cybersecurity in medical devices set by the FDA.
- Enhanced patient safety: Protect patient information while preserving the integrity and functionality of medical devices.
The Process of FDA Penetration Testing
FDA regulations for medical devices require several critical steps for penetration testing to ensure security evaluations are performed.
Planning and Preparation
- Scope Definition: Outline clearly which parts of the device will be tested.
- Testing Methodology: Determine and specify what the penetration testing approach entails: external, internal, wireless, and physical testing.
Execution of the FDA Penetration Testing
- Vulnerability Detections: Use automated tools and manual techniques to identify possible security flaws.
- Exploitation: Attempt to exploit the already identified vulnerabilities to rate the importance of security breaches.
- Analysis and Reporting: Analyze the results and document them in detailed reports for further action.
Remediation and Re-Testing
- VM: Fix the security flaws by applying security patches or changing device configurations.
- Validation Testing: Retest the device to determine that all identified vulnerabilities have been mitigated.
Best Practices for FDA Penetration Testing
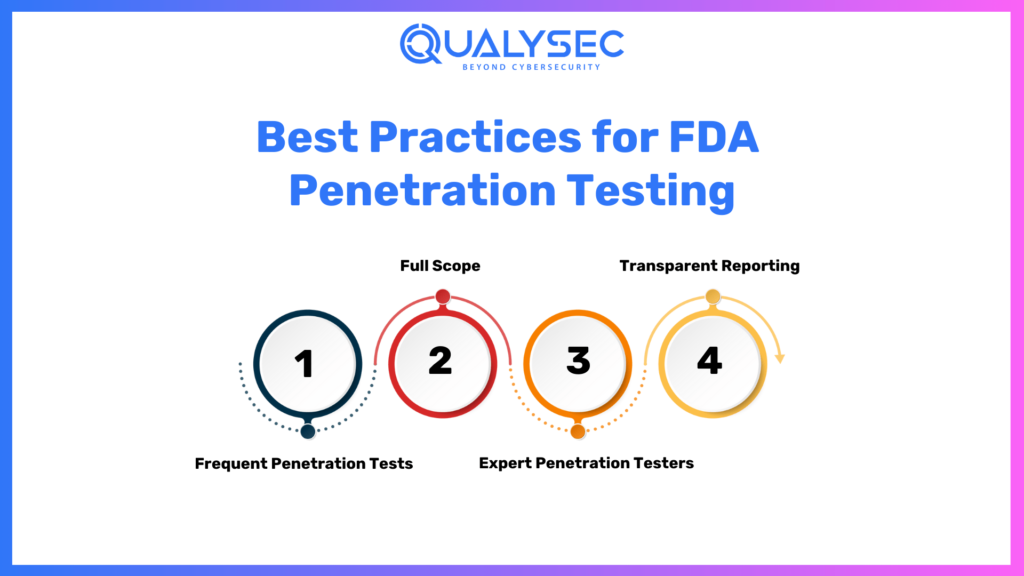
Here are the best practices for FDA Penetration Testing:
1. Frequent Penetration Tests
Penetration testing should not only be carried out during the FDA clearance application phase but also periodically during a device’s lifecycle to maintain a continuing sense of security.
2. Full Scope
Be sure to test as many access points and use cases as possible, to identify and remediate all potential vulnerabilities.
3. Expert Penetration Testers
Contract with seasoned cybersecurity professionals who understand both penetration testing methodologies and the FDA regulatory guidelines.
4. Transparent Reporting
Maintain a clear and detailed reporting practice, which will inform stakeholders and regulatory bodies regarding the testing process and results.
Benefits of FDA Penetration Testing
Below are the benefits of FDA penetration testing:
- Improved Device Security
Penetration testing enhances the security of medical devices through the identification and mitigation of vulnerabilities, thereby enabling them to maintain immunity against possible cyber threats.
- Regulatory Compliance
This means penetration tests for manufacturers to pass FDA cybersecurity standards for market approval and maintaining device legality.
- High Patient Confidence
The commitment to rigorous security testing indicates a manufacturer’s commitment to the safety of patients, encouraging belief in their products.
Latest Penetration Testing Report

FDA Penetration Testing Tools and Techniques
Here are the FDA penetration testing tools & techniques:
Automated Tools
- Nmap: Network discovery as well as security auditing.
- Metasploit: Exploit code development against the target machine executing.
- Burp Suite: web application security testing.
Manual Techniques
- Code Reviews: Manual examination of the source code for the device and to find potential security flaws.
- Security Audits: Comprehensive reviews of the device’s security posture, including configurations and operational procedures.
Wireless Testing
- Signal Interception: Testing the device’s resistance to wireless signal interception and jamming.
- Protocol Analysis: Examining communication protocols for vulnerabilities.
The Basics of 510(k) and PMA Submissions
510(k) and PMA are the two submissions through which medical device manufacturers seek FDA approval. Devices with 510(k) pathways are substantially equivalent to those already in the market, whereas PMA follows devices that are novel or significantly different from those already existing. Both, however, require extensive information about the device that the manufacturer must provide, including safety data, performance testing, and clinical evidence to establish that it is safe and effective for use by human patients.
In the 510(k) pathway, which encompasses principally Class II devices, the manufacturer is to prove, primarily, that the product is as safe and effective as a legally marketed predicate device. This means an adequate degree of risk control for the new device. On the contrary, the PMA pathway claims robust a product featuring a higher-level risk or novelty and usually faces more extensive review.
Eligibility criteria for 510(k) clearance
To obtain 510(k) clearance, medical devices must comply with predefined criteria identified and briefly summarized below.
- Device Risk Level— As an impassioned balance of safety and efficiency, the FDA has established 3 levels of oversight based on the risk each device poses. They are, broadly, low-risk devices exempt from premarket submission, medium-risk devices or Premarket Notification or 510(k), and high-risk devices, including Premarket Approval (PMA).
- Low—risk devices are generally exempt from premarket notification and include most Class I and select Class II devices.
- Medium—risk devices are mostly Class II devices, with some Class I devices. These require a 510(k).
- High—risk devices are those that sustain or support life, are implanted, or present a potentially unreasonable risk of illness or injury. These require premarket approval.
- Device Type — The types of devices that are not eligible for the 510(k) clearance process are: Devices that will be used as part of another device; Custom devices, and Devices intended for investigational use.
- Device History — The device must not have a history of being banned or withdrawn from the market; the FDA imposed any restriction on its use.
- Device Characteristics — The device must not pose an unreasonable risk to public health or safety and must be manufactured according to the 21 CFR 820 Quality System Regulation (QSR). It also has to comply with any appropriate performance standard that could be developed by the FDA or other international standards organizations. Major in scope, the device must be “substantially equivalent” to a legally marketed medical device, sometimes referred to as a “predicate device,” that was already cleared by the FDA.
Key requirements for a successful 510(k) submission
Several things must come together for a successful 510(k).
Some of these are pretty obvious and follow directly from the eligibility criteria discussed above. Others are less apparent, making the value of engaging the services of a savvy regulatory consultant who understands precisely what the FDA is trying to get at and can document details to their satisfaction on day one hard to overstate.
- Understanding of the Device Classification —Device manufacturers have to determine the proper device classification along with its applicable regulatory requirements. This is to be determined first in deciding on the proper kind of 510(k) to submit.
- Proper documentation of the device design with proper work and design will be a part of the 510(k) submission. The documentation must, as a minimum, provide drawings, schematics, and an intended use and performance description of the device.
- Selection of the most appropriate predicate device — The 510(k) submission shall include a comparison of the device under consideration to a predicate device already on the market. The predicate device selected must be of similar design, intended use, and technology.
- Demonstration of substantial equivalence —1 Each 510(k) application must consist of adequate evidence convincing the FDA that the device is substantially equivalent to the predicate device and does not involve any new questions of safety or effectiveness.
- Reasonable labeling and user instructions — Reasonable labeling and user instructions have to be such as are adequate, complete, and accurate to ensure that the user will be able to use the device safely.
- Adequate testing — Adequate testing must be conducted to demonstrate compliance of the device in question with all applicable standards. Testing must be appropriate and follow the type of device and intended use.
- Complete and accurate data —The data in the 510(k) submission shall be complete, accurate, and well-organized. No submissions will be accepted by the FDA if they are incomplete, falsified, or misleadingly presented.
Cybersecurity Concerns in Medical Device Manufacturing
There are numerous cybersecurity issues regarding the manufacturing of medical devices. For example, wireless communication and internet connectivity available in devices will provide an easy way for unwanted access and be exploited by malevolent actors. The growing software and firmware of medical devices with complexity will allow hackers to potentially exploit these issues.
Another significant issue that hackers can gain entry to is manipulating the function of medical devices. Such alterations might cause dosages to change on infusion pumps with drugs, pacemakers malfunction, and others. Patients in dire need of such equipment would likely lose their lives because of these malfunctions.
How the FDA Evaluates Cybersecurity in Device Submissions
In evaluating device submissions, the FDA reviews the cybersecurity measures implemented by the manufacturers to ensure that medical devices are safe from probable threats. This includes software and firmware integrity checks, testing the efficiency of encryption and authentication mechanisms, and also a review of the manufacturer’s incident response plans concerning vulnerabilities and breaches.
It tests the cybersecurity measures implemented by manufacturers with rigorous validations. Testing also includes simulated cyber attacks to check device resilience against threats. The FDA, in testing medical devices, thereby sets tough conditions to achieve the highest standards of cybersecurity.
The FDA considers cybersecurity aspects not only from a technical angle but also from human factors. This is centered on the analysis of the training and awareness programs that device manufacturers provide their potential users and interactions with the devices. The FDA’s holistic view is to make sure that practitioners will know anything pertinent to implementing cyber security best practices.
Strategies for Incorporating Cybersecurity in Device Submissions
Medical device manufacturers seeking approval ought to follow the best practices, strategies, and processes that efficiently incorporate cybersecurity throughout the design and submission process.
Ensuring Compliance with FDA Cybersecurity Requirements
Manufacturers need to adopt certain practices that ensure their devices fulfill the FDA cybersecurity requirements to maximize approval chances. A close working relationship with the cybersecurity experts, as well as rigorous testing, evaluation procedures, and documentation of the applicable cybersecurity measures employed in the device, are helpful practices in that regard.
Through training, one feels safer to take a new approach to working together with cybersecurity experts who could provide valuable insight and guidance throughout the development and submission process. Cybersecurity experts will also help manufacturers navigate a complex and ever-changing landscape of cybersecurity regulations and how their devices should comply.
That done, the next steps prove the efficiency of installed cybersecurity measures: penetration testing, FDA 510(k) vulnerability assessment, and code review. Such a process looks at ways that might be exploited. Fixing, for instance, vulnerabilities ahead of a presentation to the FDA would give the manufacturer a better chance to win FDA approval.
How Qualysec Can Help?
Qualysec has specialized services dedicated to FDA penetration testing. You can rest assured that your medical devices are compliant with the highest cybersecurity standards. Our experience in cybersecurity allows us to apply our knowledge to the latest regulatory requirements from the Food and Drug Administration.
Advanced tools and techniques assist us in pinpointing vulnerabilities. We assist customers in an end-to-end process from initial planning and scoping to remediation and re-testing-or in the name of compliance and security. The adoption of Qualysec enables one to improve the security profile, ensure compliance with regulations, and win customers’ confidence through secure and robust medical devices.
Talk to our Cybersecurity Expert to discuss your specific needs and how we can help your business.
Conclusion
Cybersecurity is one of the fundamental components of FDA approval of medical devices. The regulation specifies that manufacturers are expected to include cybersecurity considerations in their 510(k) and PMA submissions to address potential risks and protect patients from harm caused by medical devices that are vulnerable to cyberattacks. Familiarity with FDA approval, the intersection of cybersecurity and medical device approval, and thereby strategic compliance with cybersecurity factors constitute a big win for FDA penetration testing submissions and enhance security overall for medical devices.
As the industry of medical devices evolves, so does the great necessity of having robust cybersecurity measures in place throughout the process of FDA approval. Qualysec acknowledges the challenges that surround the cybersecurity of medical devices and assists clients with FDA compliance in a stress-free manner. The company specializes in penetration testing, HIPAA compliance, and ensuring the security of your devices to the highest levels of standards.
We are a veteran-owned company dedicated to protecting your products while securing your business against cyber threats. Call Qualysec experts today for assistance with cybersecurity and partner with a team that is as passionate about your safety as you are about healthcare innovation.
Ensure your healthcare solution is globally compliant.
Qualysec helps you meet HIPAA, FDA, ISO, and more. Contact us today!






















































































































































































































































































0 Comments