Medical devices are flourishing and expected to grow exponentially in the coming days, with global market estimates touching $799 billion by 2030 on the back of AI, robotics, and digital health (Fortune Business Insights, 2023). Where innovation is improving patient care, it also makes stringent regulatory vigilance imperative toward safety, efficacy, and adherence. Most commonly obtained in the United States, the FDA 510(k) Compliance notification is a route for moderate-risk medical devices because it allows a manufacturer the ability to prove substantial equivalence with an already accepted product.
Mastering the process of 510(k) can be of absolute importance in the production of medical devices for eventual entry into the market as early as possible, strictly under the standard of regulatory affairs. Non-compliance would attract delays, fines, or recall of products due to their business processes and patient welfare being overtly disrupted.
This article discusses the FDA 510(k) premarket, which most manufacturers view as a critical regulatory requirement, especially in ensuring market success and international credibility in the medical device industry.
What Is FDA 510(k) Compliance?
FDA compliance is said to be that process that lets a medical device manufacturer bring his product into the market by demonstrating that it is substantially equivalent to an existing predicate device. A predicate device refers to a medicare device marketed legally and subjected to the Food and Drug Administration’s review and clearance process before its marketing and use. Devices in class II and those in class I that are held to pose lesser risks to a patient fall generally under the pathway of 510(k).
Where the Premarket Approval process requires significant clinical trials to support the safety and efficacy determination, the 510(k) process can speed up regulatory clearance by demonstrating the comparability of a device’s safety and effectiveness to an approved predicate device.
That should save time and money, normally making it the path of least resistance for most medical device companies. When Should a 510(k) be filed?
Some 510(k) submission scenarios are mentioned below:
1. Introducing New Device: A firm designed a new device that was not approved by the FDA before but almost looks like another that was approved before.
2. A significant change in a marked device design, material, technology, use, or manner of manufacture that is likely to result in a significant change in safety and performance, submitted for the first time.
3. Re-entry of a product already marketed: A product sold or transferred is taken out or removed from marketing; a new 510(k) is submitted before readmission.
At this point, if the FDA is satisfied by the device’s substantial equivalence during the review, it will issue 510(k) clearance, which will allow a marketer to market and sell legally in the United States.
Why is FDA 510(k) compliance important?
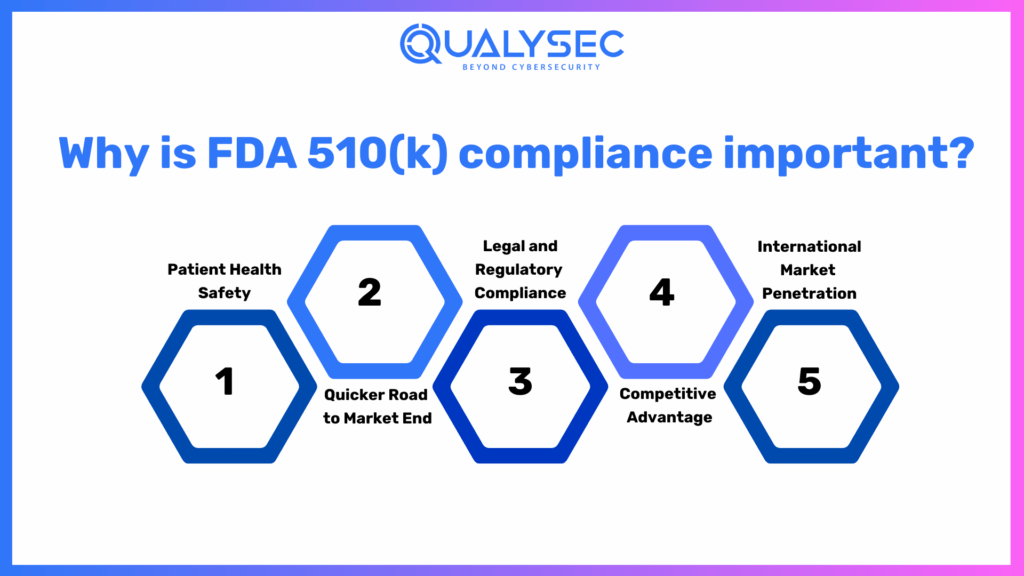
FDA compliance consultants clearance, indeed, represents one of the most crucial U.S. federal regulatory requirements that ensure a medical device meets all the areas of safety, effectiveness, and quality before the product is released in the U.S. market. Non-compliance issues can lead to legal suits, recalls, and reputational damage, therefore making the call for demand from manufacturers. Several research papers and even real-world case examples underline the relevance of these aspects in why 510(k) clearance is important: protect patient health, accelerate market entry, and provide support for global growth.
1. Patient Health Safety
Since medical devices come into direct contact with human health, they must be safe. Therefore, the mechanism of protection through the 510(k) process contains the component of significant equivalence with those that existed prior to and have FDA compliance medical devices approval. Thus, this gives way to fulfilling the strictures of the FDA concerning safety and performance.
A 2021 Journal of Medical Devices study found that 97% of all devices cleared with 510 (k) went through post-marketing safety testing compliance tests, resulting in reduced adverse patient outcomes. According to the FDA’s Medical Device Safety Action Plan in 2022, “due to stricter controls implemented by them, fewer complications developed in the new devices.”
Case study: Philips Respironics Recall between 2021-2022
Philips Respironics recalled millions of CPAP and BiPAP masks in 2021 due to foam degradation, which degrades sound abatement and risks toxic inhalation. The move was motivated by unanticipated safety concerns that would undermine FDA compliance services if they were not adhered to. Systems under the pathway of 510(k) pass through thorough reviews before hitting the market, resulting in fewer accidents.
2. Quicker Road to Market End
The 510(k) process is much faster and less expensive than the PMA process because it uses clinical trials. According to statistics, the time to market for a product that gets a 510(k) clearance is 6 to 9 months, while that for the PMA takes 3 to 7 years (Regulatory Affairs Journal, 2023). This is essential to having quick releases of innovations in the field to health providers.
Case of Wearable Glucose Monitors
The past few years have brought a new revolution of wearable continuous glucose monitors (CGMs) for diabetes management. Dexcom and Abbott introduced new models of CGMs through the 510(k) pathway, resulting in less time for the approval process and, therefore, faster adoption in the clinic. Abbott’s Freestyle Libre system was cleared by the 510(k) in 2017 and opened the door for many patients to non-invasive glucose monitoring.
3. Legal and Regulatory Compliance
If the firm does not successfully obtain 510k medical device clearance, it is liable to a heavy penalty. This includes but is not limited to:
- Penalties and litigations
- Shut down manufacturing
The FDA has been very stringent in its actions over the last few years, with more than 3,500 warning letters sent out in 2022 regarding non-compliant medical devices (FDA Enforcement Report, 2023).
Case Study: Theranos Scandal
Theranos is a biotechnology company that recently filed for bankruptcy after its device, Edison-blood testing, hit the markets without FDA compliance. Nonconformity of 510 (k) and misleading claims led to thousands of suits, financial charges, and the company’s liquidation in 2018. Such a case could aptly delineate the significance of regulatory compliance for business ethics.
4. Competitive Advantage
The acceptance and implementation of this device would be immediate if it is cleared, and businesses would gain in areas such as:
- They attract investors and business partnerships
- Increase distribution agreements.
- Enhance consumer confidence and brand reputation
Case Study: Robotic Surgical Systems
Companies like Intuitive Surgical, the da Vinci system, and Medtronic reached the first position in the robotic-assisted surgery market through the FDA clearance process. The FDA cybersecurity guidance has assured hospitals, thus encouraging them to invest their resources in robotic surgery technology, which currently receives wide coverage.
5. International Market Penetration
Examples of the nearly universally accepted FDA 510(k) clearance include:
- European Union (CE Marking process)
- Canada (Health Canada licensing)
- Australia (Therapeutic Goods Administration – TGA)
Obtaining FDA approval often results in approval in the rest of the world sooner than might otherwise be expected, with significant ease in the commercialization process in other territories.
Case Study: AI-Powered Imaging Devices
For example, in 2018, FDA 510(k) clearance was granted to AI-based diagnostic tools like Viz.ai stroke detection software. FDA approval led to expedient market adoption in the EU and Canada, and Viz.ai has become a worldwide leader in AI-driven diagnostics.
FDA 510(k) Submission Procedure
Step 1: Determine if a 510(k) Submission Is Required
- Manufacturers must determine if their device is one of the following:
- An example of a Class I, II, or III product (most Class I products exempt).
- Substantially equivalent to an existing predicate device.
- A new type or major modification to an existing type of device.
Step 2: Choose a Predicate Device
A predicate device is an FDA regulation for medical devices that are substantially equivalent to the following:
- Its intended use,
- Technological design,
- Performance parameters
Step 3: Conduct Any Required Testing
The FDA compliance expert has to demonstrate that the device is safe and performs as intended. This can include the following:
- Biocompatibility testing
- Electrical safety testing
- Software validation
- Mechanical performance tests
Step 4: Assemble the 510(k) Application
A 510(k) application has
1. Device Description – What the device does and what it is intended to do.
2. Predicate Device Comparison – Demonstrating substantial equivalence.
3. Performance Testing Data – Bench tests, animal studies, or clinical data.
4. Risk Analysis and Mitigation – Introduces possible safety issues.
5. Labeling and Instructions for Use – Meets the FDA requirement on labeling
Step 5: Submission to FDA
The 510(k) submission is submitted to the FDA for review. The standard review period is 90 days, though requests for information can extend the review period beyond that.
Step 6: Obtain FDA Clearance
When the FDA deems a device to be substantially equivalent, it issues a 510(k) clearance letter, enabling the manufacturer to market the device legally.
Obstacles to Obtain 510(k) Clearance
Although the FDA regulatory guidelines process is beneficial, there are many hurdles:
1. Choosing the Predicate Device
Finding a suitable predicate device may not be easy, particularly for innovative technologies. This can result in delay or rejection.
2. Shifts in FDA Requirements and Compliance
The FDA issues new requirements very often. If the new requirements change over time, there has to be a shift in compliance for that manufacturer.
3. Requirements in Data and Testing
Failure to perform substantial equivalence testing may cause further delay in the review and could even warrant another round of data requests by the FDA.
4. Substantial Equivalence Demonstration Problems
Substantial equivalence may be difficult to establish when the device offers new features or advanced technology. The FDA may demand more justification or clinical data.
New Trends and Innovation in 510(k) Compliance
1. FDA’s Safety and Performance-Based Pathway
The FDA ramped up its Safety and Performance-Based Pathway in 2019, allowing manufacturers to demonstrate equivalence based on predetermined performance criteria, not predicate devices.
2. Digital Health and AI-Powered Devices
The FDA evolved a new rule in machine learning models and software as a medical device in AI-based applications for medical devices.
3. RWE (Real-World Evidence)
Real-life clinical data will also be the lifeline of the FDA for more 510(k) submissions, which will make 510(k) more dynamic and ever-changing.
4. Greater Transparency with Public Databases
This made the 510(k) database so accessible that manufacturers can quickly trace down predicate devices now.
How Manufacturers Can Ensure 510(k) Success
To ensure a product that will likely clear, manufacturers need to remember:
- Pre-design analysis
- The right predicate device is used to ensure comparability through strong data.
- Good testing and documentation to minimize additional data requests.
- Stay updated on the FDA’s rules and regulation network with experts.
- Plan for delays and be able to make adjustments in your regulatory strategy.
Conclusion
FDA device approval is both a legal necessity and a corporate strategy to secure market penetration for any type of medical device company. Therefore, by providing proof of reasonable equivalency, credibility can be built. Cases and research have proven that 510(k)-cleared devices reach the market sooner, yielding better outcomes for patients as opposed to other products, reducing the chances of facing regulations.
While these are considered legal, there’s still an underlying complexity involved with navigating FDA regulations, preparation of detailed documentation, and continuous compliance. The chance of errors at the time of submission leads to delays, penalties, or recalls, all of which may harm the business and the marketplace.
It is here that Qualysec, the leading cybersecurity and compliance testing company, understands, from its knowledge of regulatory assessments, risk analysis, and quality assurance, that medical device manufacturers can confidently meet all the requirements of FDA 510(k). Several compliance solutions for companies prevent costly regulatory setbacks, enhance security, and speed up a product’s approval.
If you are a medical equipment manufacturer seeking to easily submit your product to 510(k) and ensure regulatory success, simply contact Qualysec. Their compliance experts will walk you through the process and minimize the risks while maximizing the market potential.
Call Qualysec today to ensure that your medical device meets safety and regulatory standards when entering the U.S. market.
Ensure your healthcare solution is globally compliant.
Qualysec helps you meet HIPAA, FDA, ISO, and more. Contact us today!










































































































































































































































































0 Comments